07 Nov Living heart valve replacements
This blogpost describes part of the work our new colleague Elmer performed during his time at the Eindhoven University of technology as part of his PhD project.
Living valve replacements
Our hearts are the engine of the cardiovascular system, pumping around enough blood to provide the entire body with sufficient oxygen and nutrients to enable us to live our daily lives. Within our hearts there are four valves that ensure that the blood keeps flowing in the right direction. Two of these valves consist of three flap-like structures, called leaflets, that are apart when the blood is leaving the heart but come together to prevent backflow from the artery into the heart. When these valves get diseased, repair is often impossible and surgical replacement is required. Currently, most of the available heart valve replacements are non-living and therefore cannot adapt to changes in the patients’ needs. Living heart valve replacements will not have these disadvantages and will potentially improve the lives of young patients greatly. Currently, surgeons may create a living valve replacement using the ross procedure, replacing the diseased valve on the high-pressure side of the heart with the patients own healthy valve from the low-pressure side. For the future, a technique called tissue engineering is being developed to stimulate the patient’s own cells to create a new heart valve around a biodegradable scaffold.
A material subroutine to understand and predict growth of living heart valve replacements.
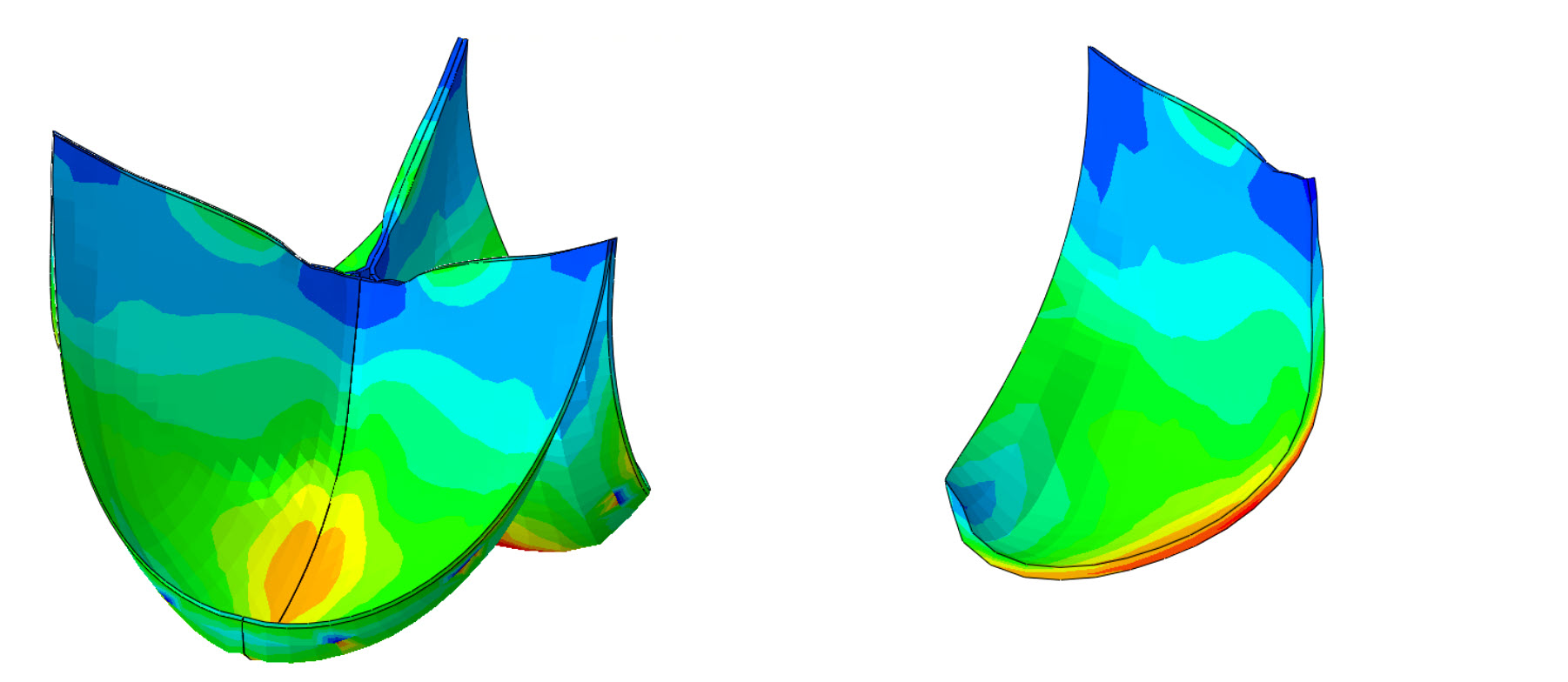
In both these procedures, an enormous mechanical demand is placed on the living heart valve replacement. This mechanical demand is sensed by the cells and stimulates various biological mechanisms to produce new and remodel the existing tissue. These growth and remodeling responses need to be fully understood before the safety of these tissue engineered heart valves can be guaranteed. To do this, an Abaqus user defined material model (UMAT) was developed that describes the mechanical behavior of the highly anisotropic and strain-stiffening heart valve tissue and biodegradable scaffold. However, the main advantage of this UMAT was that it was able to describe more than only the mechanical behavior of the valves at any given point in time, but also predicted how the tissue grew and evolved over time based. This growth and remodeling response was based on several insights in the mechanobiology of the cells within the heart valve and its consequences were integrated directly in the material model such that a predicted change in tissue mass directly induced a volume change in the finite element and a change in the tissue composition directly affected the mechanical stiffness. With these models a wide array of heart valve growth and remodeling phenomena could be investigated.
Understanding the adaptation of living valve replacements after the Ross procedure.
To deepen the understanding of the adaptation of (low pressure) pulmonary valves in the (high pressure) aortic outflow tract the Ross-procedure was numerically simulated in Abaqus. The increase in pressure induced higher stresses in the leaflet which in turn drove the cells to locally increase tissue production and turnover. This enabled us to predict local increases in the tissue thickness and composition in agreement with findings from explanted ross-valves. This animation shows the simulated geometric changes in these transplanted valves because of the tissue growth and adaptation in the months after the Ross-procedure. In addition, our numerical approach allowed us to vary several biological parameters that allowed us to explain that valves containing cells that react more strongly to a mechanical stimulus are more likely to remain functional compared to valves with a lesser response to mechanical stimulation, which often developed a dysfunctional degree of dilation. More details on the simulated growth and remodeling of Ross-autografts are available in https://link.springer.com/article/10.1007/s10237-024-01874-y
Optimizing the growth and remodeling of engineered heart valves.
Much research is undertaken to induce the patients’ body to engineer their own replacement valves directly at the functional site. Different proof of concept studies demonstrated that this can be done in several ways. One of these methods is grow a preliminary tissue valve in the lab, which is then cleared of cells and other proteins that can inflict an adverse immune response and subsequently implanted in the patient. Here it is re-infiltrated by host cells and will grow and remodel according to the needs of the patient. During this culturing phase in the laboratory, many different combinations of stimulation can be applied to steer and control the tissue-formation. However, this is a time-consuming process and will become costly to optimize using experimental means. To overcome this, the material models were extended to capture both the period of tissue culture in the laboratory phase and the subsequent growth and remodeling response in the body. This way, it is possible to numerically test different methods of tissue culturing in and monitor how this affects the subsequent viability of the valve in the body in a time- and cost-effective manner. Although this work is still ongoing, the preliminary results describe the experimental findings well and reveal that small changes in the laboratory phase may indeed be leveraged to steer the formation of the new tissue and therefore determine thelong-term success or failure of the tissue engineered valves in the body.
With this blogpost we hope to have shown you that the utilities of Abaqus extend way beyond the realm of conventional engineering materials. Are you interested to know if one of our products can also be utilized for your (unconventional) engineering application? Or do you want our engineering service to help you to develop your own user subroutines? Contact us at sales@4realsim.com!


